Describe Ionic Bond in Your Own Words
What is ionic bond in your own words. A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.

Ionic Bond Electrovalent Bond Definition Properties Electronegativity Examples With Videos
The electrostatic force of attraction between cations and anions are called ionic bond.

. Use specific examples in your answer. Covalent compounds usually have lower melting and boiling points than ionic. A chemical bond formed between two ions with opposite charges.
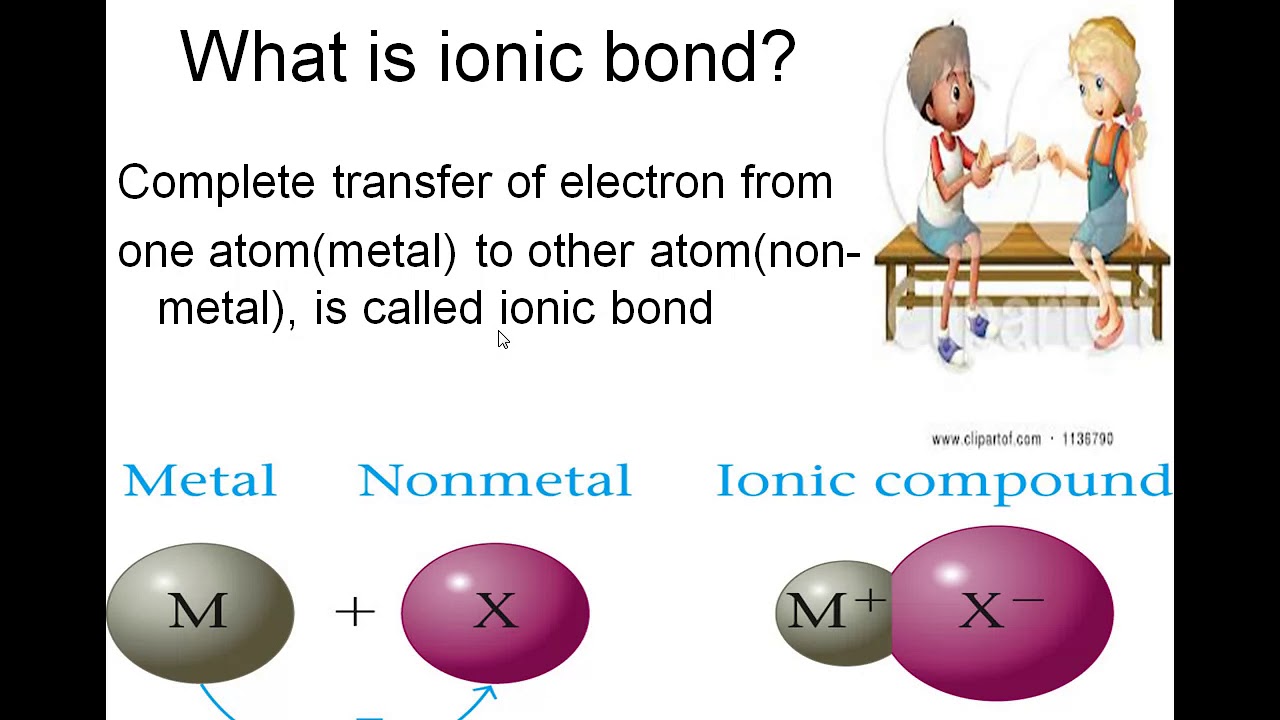
These bonds can form. A ionic bond Is the complete transfer of valence electron s between atoms. Where are metal atoms located on the periodic table.
Most covalent compounds consist of nonmetals bonded to one another. Movement of electrons from one element to another. Each atom contributes an equal number of electrons towards the bond formation.
Describe in your own words the structure of solid sodium chloride and explain why its formula is NaCl. Ionic bonds form when one atom gives up one or more electrons to another atom. A polar bond is formed by the attraction between oppositely-charged.
In your own words describe what an experimental group is. What subatomic particles participate in chemical bonding. View OL Lab 4-Ionic and Covalent Bondsasd lab 4docx from CHEM 1411 at Eastfield College.
How is the naming of ionic and covalent compounds different. In your own words describe what a Control group is. 1 Ionic bond.
In your own words explain the terms below. For the most part ionic compounds contain a metal bonded to a nonmetal. Both types involve multiple atoms coming together to form a more complex structure.
Ionic Bond Vs Covalent Bond. In other words the electron spends most of its time close to the bonded atom. In your own words define valence electron.
Predict the formula and find the number of lattice points when atoms of A are present at corner and B are present at face centrethankyou. An example of an ionic bond is the chemical compound Sodium Chloride. So then sodium ions will attract chloride ions and form an ionic bond.
NaCl or table salt. Steady state of relative reactant and product concentration in reversible chemical reactions in a closed system. Start your trial now.
Similarities between Ionic and Covalent Bonding and Compounds. Covalent bonds are formed between two non-metals whereas ionic bonds are formed between a metal and non-metal. In an experiment you have an Experimental Group.
The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. Metals form cations by loosing electrons and. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Up to 24 cash back 1. It is a type of chemical bond. Ionic Covalent Virtual Lab.
Students should become more familiar with how to predict charges of ions in an ionic bond during this simulation. Define equilibrium in your own words. You may include other examples and other supporting information to make your points clear and accurate.
Opposite charges attract right. In your own words define. Be sure to refer to valence electrons in your response.
The result is a. Analyse the composition of each compound to classify the type of chemical bonding. The covalent bond is a bond formed when two atoms share one or more electron pairs.
In your own words write a sentence showing the relationship between ion and ionic bondpls solve for me its my homework Why is fusion only possible with small nuclei. Ionic bonds form when one atom gives up one or more electrons to another atom. Ionic bonds form when one atom gives up one or more electrons to another atom.
The ability to combine and to chemically bond with each other. The reaction components of covalent bonds are electrically neutral whereas for ionic bonds they are both charged. This simulation is intended to display bonding at a particle level so the user can identify the transferring of electrons in an ionic bond versus the sharing of electrons in a covalent bond.
Solution for Define the terms covalent bond and ionic bond in your own words. Ionic Bonds. Answer the questions below.
A covalent bond also called a. In both types bonding yields a compound that has different properties than the original elements. Bond between a metal and nonmetal.
Olaribigbe Matesun OL Lab 4. Bond between two nonmetals. Atoms that participate in an ionic bond have different electronegativity values from each other.
Where are non-metal atoms located on the periodic table. Weve got the study and writing resources you need for. And indicate which one is positive and which is negative - Describe a.
In your own words use the information in the picture to DESCRIBE the formation of ionic bonds using scientific words. In an ionic bond one atom essentially donates an electron to stabilize the other atom. These bonds can form between a pair of atoms or between molecules and are the type of bond found in salts.
For each of the below predict the expected ion each could become by giving the charges. What are the differences between ionic and covalent bonds. Important in creation of ionic bonds.
In your own words explain the terms below. Based on your knowledge of ionic and covalent compounds complete the missing portions of the table below. Up to 24 cash back 1.
In covalent bonds atoms share electrons whereas in ionic bonds atoms transfer electrons. By the way chloride is the term used to designate the anion form of chlorine. Where are metal atoms located on the periodic table.
Answer these before going to the website to begin the virtual lab. Where are non-metal atoms located on the periodic table. A chemical bond formed between two ions with opposite charges.
Describe the difference between an atom and a molecule. CHEM120 OL Week 2 Lab Name. Ionic-bond meaning The definition of ionic bond is when a positively charged ion forms a bond with a negatively charged ions and one atom transfers electrons to another.
Describe an ionic bond in your own words. Li 1 cation c. What is covalent bond in your own words.
Ionic compounds form crystals typically have high melting and boiling points are usually hard and brittle and form electrolytes in water. First week only 499. 2 See answers Advertisement.
Mg 2 cation d. What subatomic particles participate in chemical bonding.

Ionic Bond Definition Properties Examples Facts Britannica
No comments for "Describe Ionic Bond in Your Own Words"
Post a Comment